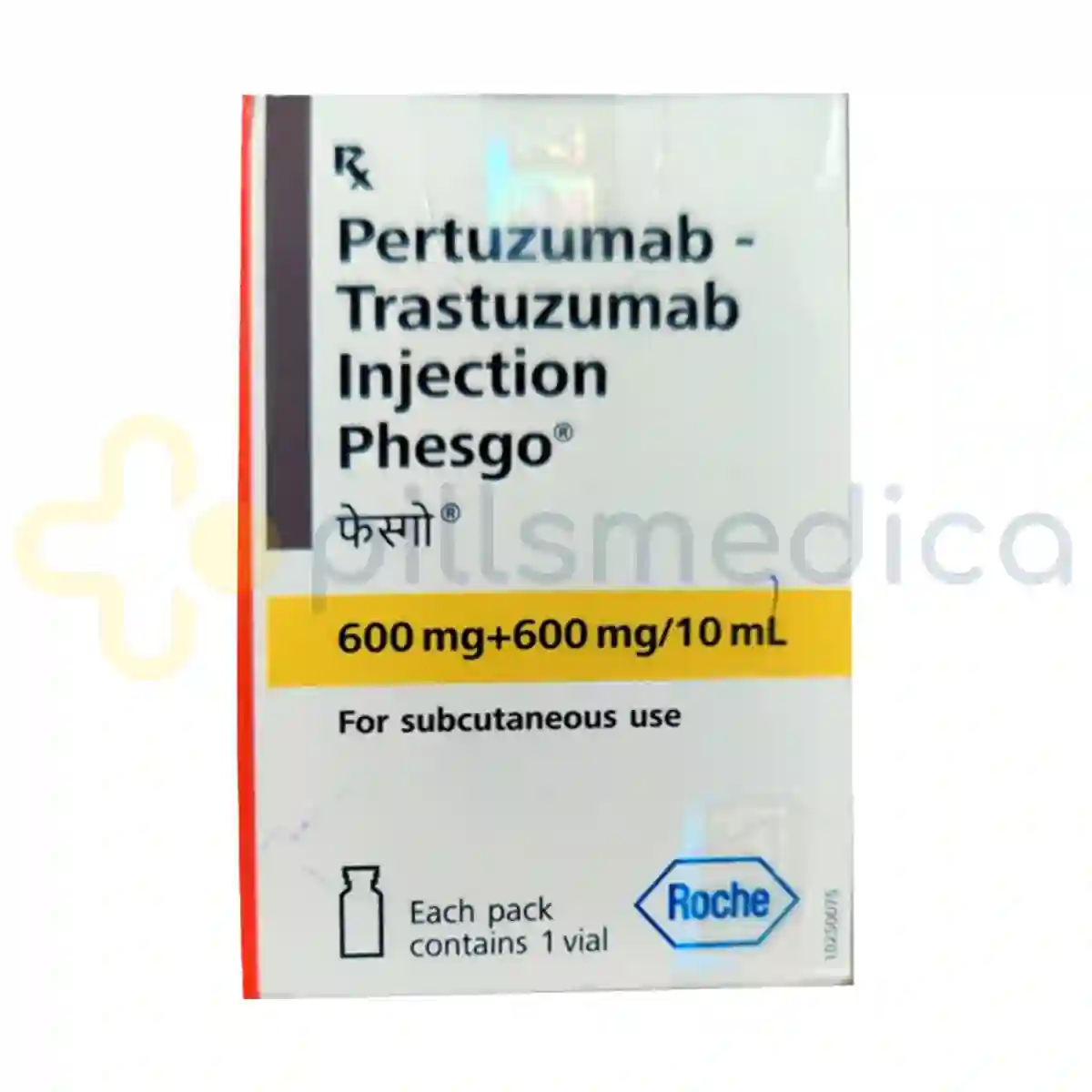
Phesgo Injection (10ml)
Phesgo Injection (10ml) is a targeted therapy used primarily in the treatment of certain types of breast cancer, specifically HER2-positive breast cancer. This innovative medication combines two monoclonal antibodies, Pertuzumab and Trastuzumab, each at a dosage of 600mg, to effectively inhibit the growth of cancer cells that overexpress the HER2 protein. By blocking the HER2 receptor, Phesgo helps to slow down or stop the proliferation of cancer cells, making it a vital component of treatment regimens for patients with this aggressive form of cancer. The injection is administered subcutaneously, providing a more convenient option compared to traditional intravenous therapies, which can enhance patient compliance and comfort. Phesgo is typically used in conjunction with chemotherapy to maximize its effectiveness, and its formulation is designed to ensure optimal absorption and therapeutic action.
Essential Drug Facts
| Dosage form | Iv(Intravenous) Injection |
|---|---|
| Habit Forming | No |
| Systemic Category | Oncology |

What are the Indication / Medical Uses of Phesgo Injection (10ml)
Phesgo Injection (10ml) is indicated for the treatment of adult patients with HER2-positive breast cancer, both in the early stages and in metastatic settings. It is particularly beneficial for those who have not received prior anti-HER2 therapy or chemotherapy for their metastatic disease. The drug is often used in combination with chemotherapy agents to improve overall treatment outcomes, making it a crucial option for patients facing this challenging diagnosis. Its use is guided by specific clinical criteria, ensuring that it is administered to those who will benefit the most from its targeted action against HER2-positive tumors.

How Does Phesgo Injection (10ml) Benefit You?
The benefits of Phesgo Injection (10ml) for patients are significant, as it offers a targeted approach to treating HER2-positive breast cancer. By specifically targeting the HER2 protein, Phesgo can effectively inhibit tumor growth and improve overall survival rates. The subcutaneous administration of the drug allows for a more convenient treatment experience, reducing the time spent in healthcare settings compared to intravenous therapies. Additionally, the combination of Pertuzumab and Trastuzumab enhances the therapeutic effect, providing a more robust defense against cancer progression. Patients may experience fewer side effects compared to traditional chemotherapy, leading to an improved quality of life during treatment.
.svg)
Phesgo Injection (10ml) Action Mechanism
Phesgo Injection (10ml) works by targeting the HER2 receptor, which is overexpressed in certain breast cancer cells. Pertuzumab and Trastuzumab, the active components of Phesgo, bind to different sites on the HER2 protein, preventing it from signaling cancer cells to grow and divide. This dual blockade not only inhibits the proliferation of cancer cells but also marks them for destruction by the immune system. By disrupting the HER2 signaling pathway, Phesgo effectively slows down tumor growth and can lead to a reduction in tumor size, making it a powerful option in the management of HER2-positive breast cancer.

How should Phesgo Injection (10ml) be used
Phesgo Injection (10ml) should be used as directed by a healthcare professional. The recommended dosage is typically administered as a subcutaneous injection, with the initial dose given in conjunction with chemotherapy. Subsequent doses are usually administered every three weeks. It is essential for patients to follow their healthcare provider's instructions regarding the timing and frequency of injections to ensure optimal therapeutic outcomes. Patients should also be monitored for any adverse reactions or side effects during treatment.

When is the best time to take Phesgo Injection (10ml) ?
The best time to take Phesgo Injection (10ml) is as prescribed by a healthcare provider, typically in conjunction with a chemotherapy regimen. The initial dose is often given before the first cycle of chemotherapy, followed by subsequent doses every three weeks. It is crucial for patients to adhere to the scheduled treatment plan to maintain the drug's effectiveness and to monitor for any potential side effects. Patients should discuss any concerns regarding timing with their healthcare team to ensure the best possible outcomes.

How does it affect me if I overdose?
In the event of an overdose of Phesgo Injection (10ml), patients may experience an increased risk of severe side effects, including more pronounced adverse reactions such as heart problems, infusion-related reactions, or other serious complications. It is essential to seek immediate medical attention if an overdose is suspected. Healthcare providers will assess the situation and provide appropriate interventions to manage any adverse effects resulting from the overdose.
.svg)
How does it affect me if I miss a dose?
If a patient misses a dose of Phesgo Injection (10ml), it is important to contact their healthcare provider as soon as possible for guidance. Depending on the timing of the missed dose, the provider may recommend rescheduling the injection or adjusting the treatment plan. Missing a dose can potentially affect the overall effectiveness of the treatment, so timely communication with the healthcare team is crucial to ensure continuity of care.

Drug Adverse Reactions of Phesgo Injection (10ml)
Phesgo Injection (10ml) may cause various side effects and adverse reactions, which can range from mild to severe. Common side effects include fatigue, nausea, diarrhea, and infusion-related reactions such as fever, chills, or rash. More serious adverse reactions may include heart problems, particularly in patients with pre-existing heart conditions. It is essential for patients to report any unusual symptoms or side effects to their healthcare provider promptly to ensure appropriate management and care.

Phesgo Injection (10ml) Tablet is contraindicated in following conditions
Phesgo Injection (10ml) is contraindicated in patients with a known hypersensitivity to Pertuzumab, Trastuzumab, or any of the excipients in the formulation. Additionally, it should not be used in patients with a history of severe infusion reactions to monoclonal antibodies. Caution is also advised in patients with a history of heart disease or those who are pregnant or breastfeeding, as the safety of Phesgo in these populations has not been fully established.

Phesgo Injection (10ml) Safety Warnings and Precautions

While there are no direct contraindications, alcohol consumption may interfere with the overall health and recovery of patients undergoing treatment with Phesgo, and moderation is advised.

Patients with known hypersensitivity to Pertuzumab, Trastuzumab, or any component of Phesgo should avoid its use due to the risk of severe allergic reactions.

The use of Phesgo during pregnancy may harm the developing fetus, as both Pertuzumab and Trastuzumab can cross the placenta and potentially lead to adverse effects.

The safety and efficacy of Phesgo in pediatric patients have not been established, and its use in children should be approached with caution.

It is not known whether Pertuzumab and Trastuzumab are excreted in human milk; therefore, breastfeeding while receiving Phesgo is not recommended due to the potential risk to the nursing infant.

Phesgo may cause side effects such as fatigue or dizziness, which could impair the ability to drive or operate machinery safely, so patients should assess their individual response before engaging in these activities.

Patients with renal impairment may require dose adjustments or closer monitoring, as the effects of Phesgo in this population are not fully understood.

Patients with a history of lung disease or pulmonary complications should be monitored, as Phesgo may exacerbate respiratory issues.

Liver function should be assessed before treatment, as patients with hepatic impairment may experience altered drug metabolism and increased risk of side effects.

Phesgo can cause cardiotoxicity, and patients with pre-existing heart conditions or those receiving other cardiotoxic therapies should be monitored closely for heart function.

Interactions between Phesgo Injection (10ml)
Phesgo Injection (10ml) may interact with other medications, potentially altering their effectiveness or increasing the risk of adverse effects. Patients should inform their healthcare provider of all medications, supplements, and herbal products they are taking to avoid potential interactions. Special caution should be exercised when combining Phesgo with other drugs that may affect heart function or those that are known to interact with monoclonal antibodies.

How to store and dispose of Phesgo Injection (10ml)
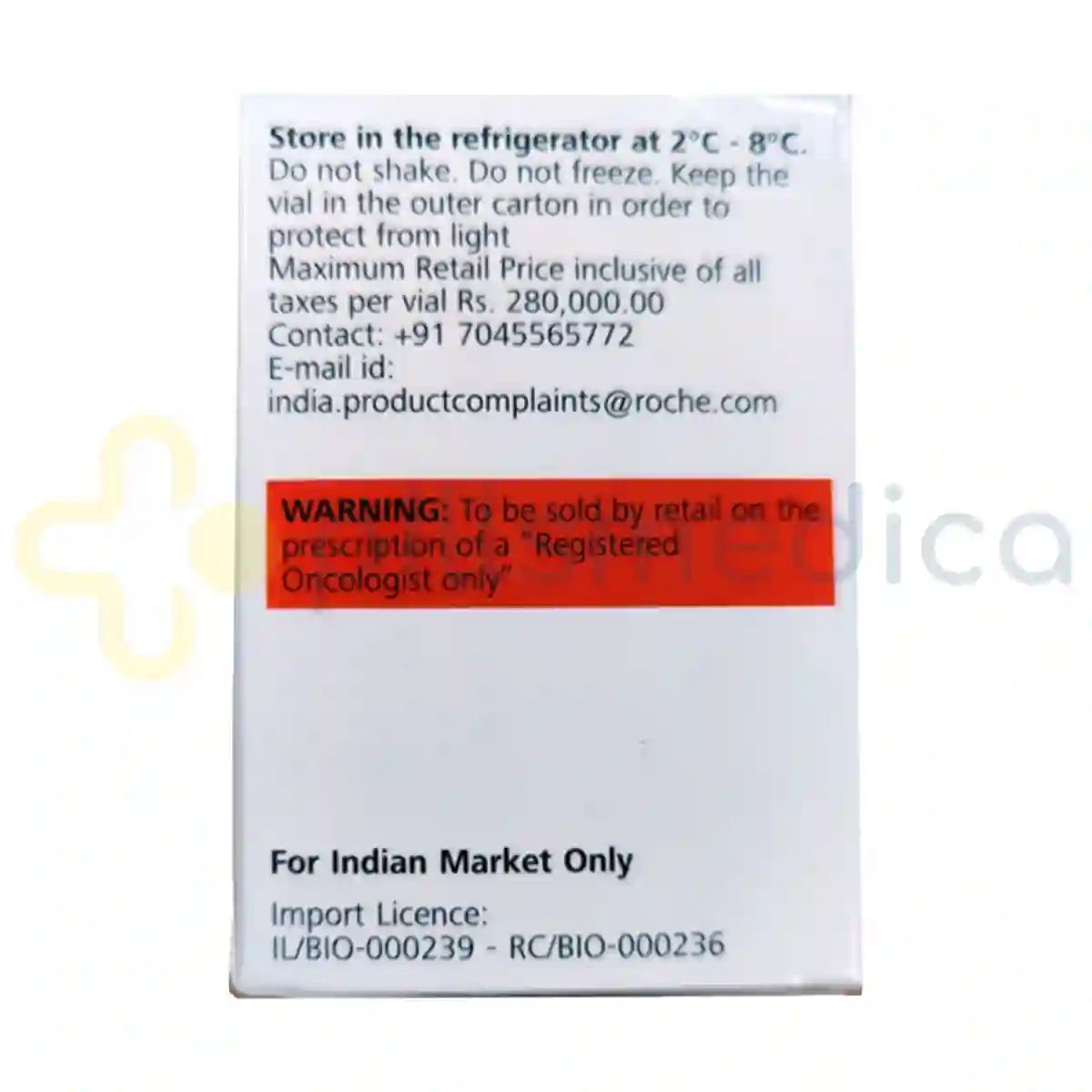
Phesgo Injection (10ml) should be stored in a refrigerator at a temperature between 2°C to 8°C (36°F to 46°F) and protected from light. It should not be frozen. Once removed from refrigeration, it can be kept at room temperature for a limited time, but it should be used within the specified time frame to ensure its effectiveness. Proper disposal of unused or expired medication should be done according to local regulations, and patients should consult their healthcare provider or pharmacist for guidance on safe disposal methods.

Here are a few quick tips for Phesgo Injection (10ml)
For effective and safe use of Phesgo Injection (10ml), patients should adhere to their prescribed treatment schedule, report any side effects or unusual symptoms to their healthcare provider, and attend all follow-up appointments for monitoring. It is also important to maintain open communication with the healthcare team regarding any concerns or questions about the treatment. Patients should ensure they are well-hydrated and maintain a balanced diet to support their overall health during therapy.

Parameters that should be monitored Phesgo Injection (10ml)
While using Phesgo Injection (10ml), healthcare providers should monitor several parameters, including cardiac function, liver and kidney function, and overall blood counts. Regular assessments through echocardiograms or other cardiac evaluations may be necessary to detect any potential heart-related side effects early. Additionally, monitoring for infusion-related reactions and managing any side effects promptly is crucial for patient safety and treatment efficacy.

Considerations related to diet Phesgo Injection (10ml)
Dietary considerations while using Phesgo Injection (10ml) include maintaining a balanced and nutritious diet to support overall health and recovery during treatment. Patients should stay well-hydrated and may benefit from small, frequent meals to manage any nausea or gastrointestinal side effects. It is advisable to avoid alcohol and limit caffeine intake, as these can exacerbate side effects. Patients should consult their healthcare provider or a nutritionist for personalized dietary recommendations during their treatment.

Question and Answer (FAQ)
Q: What is Phesgo Injection used for?
A: Phesgo Injection is used to treat HER2-positive breast cancer in adults, often in combination with chemotherapy.
Q: How is Phesgo administered?
A: Phesgo is administered as a subcutaneous injection, typically every three weeks.
Q: What are the side effects of Phesgo?
A: Common side effects include fatigue, nausea, diarrhea, and infusion-related reactions. Serious side effects may include heart problems.
Q: Can I take Phesgo if I am pregnant?
A: Phesgo is not recommended during pregnancy due to potential risks to the fetus.
Q: Is Phesgo safe for breastfeeding?
A: Phesgo is considered unsafe during breastfeeding, as it may be excreted in breast milk.
Q: What should I do if I miss a dose of Phesgo?
A: If you miss a dose, contact your healthcare provider for guidance on rescheduling.
Q: Can Phesgo interact with other medications?
A: Yes, Phesgo may interact with other medications, so inform your healthcare provider of all drugs you are taking.
Q: How should I store Phesgo?
A: Phesgo should be stored in a refrigerator between 2°C to 8°C and protected from light.
Q: What precautions should I take while using Phesgo?
A: Monitor for side effects, maintain communication with your healthcare provider, and attend all follow-up appointments.
Q: Is it safe to drive while taking Phesgo?
A: Phesgo does not typically impair driving, but assess your individual response to the medication before driving.







Reviews
There are no reviews yet.