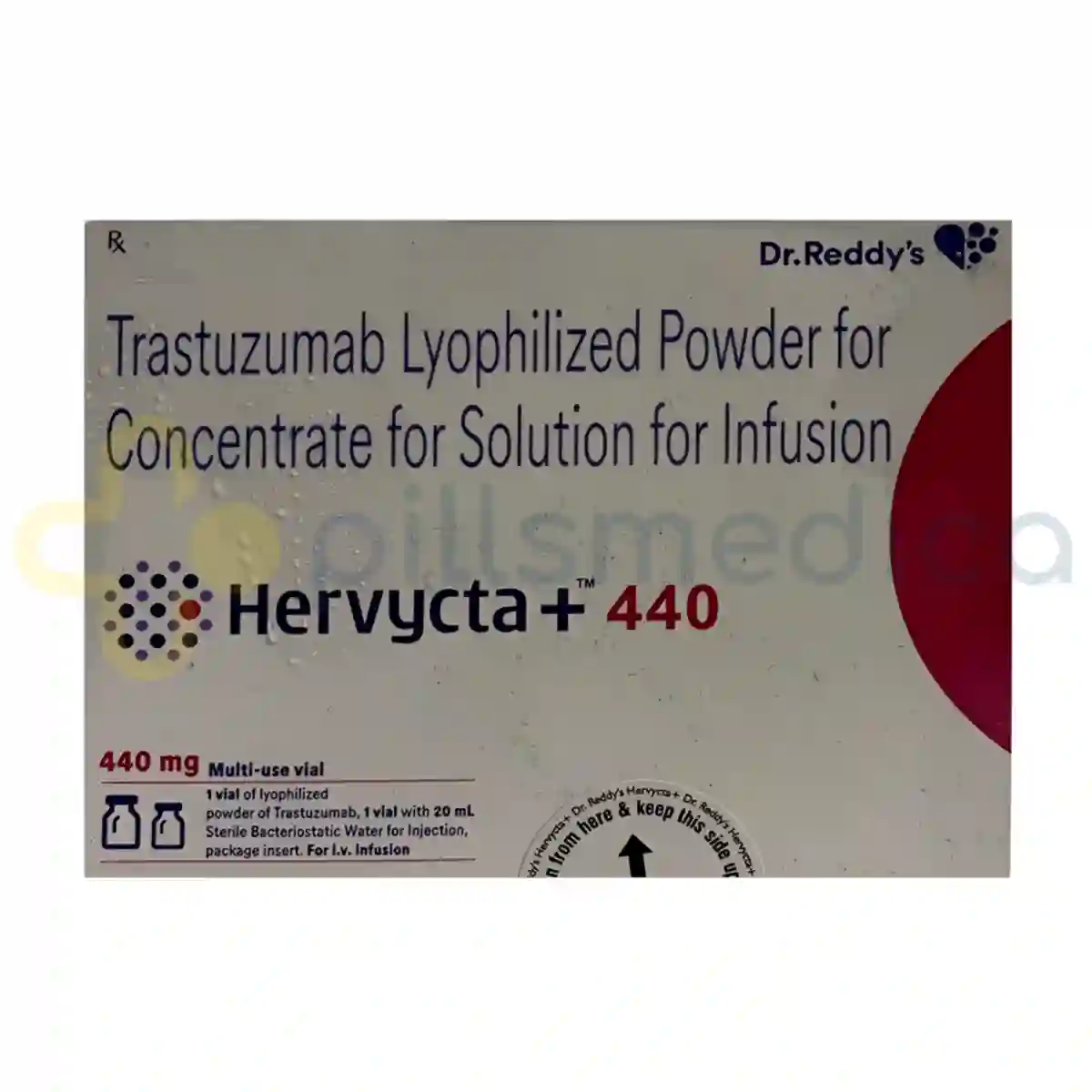
Hervycta Injection (440mg)
Hervycta Injection (440mg), which contains the active ingredient Trastuzumab, is a monoclonal antibody primarily used in the treatment of certain types of breast cancer and gastric cancer that overexpress the HER2 protein. This medication is designed to target and inhibit the growth of cancer cells that have high levels of HER2, a protein that promotes the growth of cancer cells. By binding to the HER2 receptors on the surface of these cells, Hervycta helps to block the signals that would otherwise lead to their proliferation. The injection is typically administered intravenously and is often used in conjunction with other cancer therapies to enhance treatment efficacy. Patients receiving Hervycta may experience improved outcomes in terms of tumor response and overall survival, making it a critical component in the management of HER2-positive cancers.
Essential Drug Facts
| Dosage form | Iv(Intravenous) Injection |
|---|---|
| Chemical Class | Monoclonal antibody (mAb) |
| Habit Forming | No |
| Systemic Category | Oncology |
| Action Class | HER2/neu (ErbB2) Inhibitor- Monoclonal antibody |

What are the Indication / Medical Uses of Hervycta Injection (440mg)
Hervycta Injection (440mg) is indicated for the treatment of adult patients with HER2-positive breast cancer, either in the early stages or metastatic forms, as well as for patients with HER2-positive gastric or gastroesophageal junction adenocarcinoma. It is often prescribed for patients who have not received prior anti-HER2 therapy or chemotherapy for their metastatic disease. The drug is also used in combination with other chemotherapy agents to improve treatment outcomes and is an essential part of the therapeutic regimen for patients with these specific cancer types.

How Does Hervycta Injection (440mg) Benefit You?
The benefits of Hervycta Injection (440mg) for patients include its targeted action against HER2-positive cancer cells, which can lead to a reduction in tumor size and improved survival rates. By specifically targeting the HER2 protein, Hervycta minimizes damage to normal cells, potentially resulting in fewer side effects compared to traditional chemotherapy. Additionally, the use of this medication can enhance the effectiveness of other cancer treatments, providing a more comprehensive approach to managing HER2-positive cancers. Patients may experience a better quality of life during treatment, as the drug can help control disease progression.
.svg)
Hervycta Injection (440mg) Action Mechanism
Hervycta Injection (440mg) works by binding to the HER2 receptors on the surface of cancer cells, blocking the receptor's ability to receive growth signals. This action inhibits the proliferation of cancer cells that overexpress HER2, leading to cell cycle arrest and apoptosis (programmed cell death). Furthermore, the binding of Trastuzumab to HER2 can also recruit immune cells to attack the cancer cells, enhancing the overall anti-tumor response. This dual mechanism of action makes Hervycta a powerful agent in the treatment of HER2-positive cancers.

How should Hervycta Injection (440mg) be used
Hervycta Injection (440mg) should be used under the supervision of a healthcare professional. The dosage and administration depend on the patient's body weight and the specific treatment regimen prescribed by the oncologist. Typically, the initial dose is administered intravenously, followed by maintenance doses given at regular intervals. It is crucial for patients to adhere to the prescribed schedule and to report any side effects or concerns to their healthcare provider promptly. Proper hydration and monitoring during infusion are also recommended to manage potential infusion-related reactions.

When is the best time to take Hervycta Injection (440mg) ?
The best time to take Hervycta Injection (440mg) is determined by the treatment plan established by the healthcare provider. It is usually administered in a clinical setting, and the timing may vary based on the patient's overall treatment schedule, including other chemotherapy agents. Patients should follow their oncologist's instructions regarding the timing of doses to ensure optimal effectiveness and to minimize the risk of side effects.

How does it affect me if I overdose?
In the case of an overdose of Hervycta Injection (440mg), patients may experience severe side effects, including increased risk of heart problems, infusion reactions, and other serious complications. Symptoms of an overdose may include difficulty breathing, swelling, or severe allergic reactions. It is essential to seek immediate medical attention if an overdose is suspected, as prompt intervention can mitigate potential risks.
.svg)
How does it affect me if I miss a dose?
If a patient misses a dose of Hervycta Injection (440mg), they should contact their healthcare provider as soon as possible to determine the best course of action. Depending on the timing of the missed dose, the provider may recommend rescheduling the injection or adjusting the treatment plan. Missing a dose can potentially affect the overall treatment efficacy, so timely communication with the healthcare team is crucial.

Drug Adverse Reactions of Hervycta Injection (440mg)
Possible side effects and adverse reactions of Hervycta Injection (440mg) may include fever, chills, nausea, vomiting, diarrhea, and fatigue. More serious side effects can involve heart problems, such as decreased heart function or heart failure, and infusion-related reactions, which may present as difficulty breathing, rash, or swelling. Patients should be monitored closely for these adverse effects, and any concerning symptoms should be reported to a healthcare provider immediately.

Hervycta Injection (440mg) Tablet is contraindicated in following conditions
Hervycta Injection (440mg) is contraindicated in patients with a known hypersensitivity to Trastuzumab or any of its components. It should also be used with caution in patients with a history of heart disease or those who have experienced heart failure, as the drug can exacerbate these conditions. Additionally, it is not recommended for use in patients with severe infusion reactions to previous doses of Trastuzumab.

Hervycta Injection (440mg) Safety Warnings and Precautions

While there are no direct contraindications, alcohol consumption may interfere with the overall health and recovery of patients undergoing treatment with Trastuzumab.

Patients with a known hypersensitivity to Trastuzumab or any of its components should avoid using this medication due to the risk of severe allergic reactions.

Trastuzumab may cause harm to the developing fetus, and its use during pregnancy is generally not recommended unless the potential benefits outweigh the risks.

The safety and efficacy of Trastuzumab in pediatric patients have not been established, and careful monitoring is required if used in this population.

It is not known whether Trastuzumab is excreted in human milk, and due to the potential for serious adverse reactions in nursing infants, breastfeeding is typically advised against during treatment.

While Trastuzumab does not directly impair driving ability, patients may experience side effects such as fatigue or dizziness, which could affect their ability to operate vehicles safely.

Patients with renal impairment may require dose adjustments and close monitoring, as kidney function can affect the clearance of the drug.

Patients with a history of pulmonary issues should be monitored closely, as Trastuzumab may exacerbate respiratory conditions.

Liver function should be assessed before treatment, as hepatic impairment may influence the metabolism and excretion of Trastuzumab.

Trastuzumab can cause cardiotoxicity, particularly in patients with pre-existing heart conditions or those receiving other cardiotoxic therapies, necessitating regular cardiac monitoring.

Interactions between Hervycta Injection (440mg)
Hervycta Injection (440mg) may interact with other medications, potentially altering their effectiveness or increasing the risk of side effects. Patients should inform their healthcare provider of all medications, supplements, and herbal products they are taking to avoid potential interactions. Special caution should be taken with other drugs that may affect heart function or those that are metabolized by the liver.

How to store and dispose of Hervycta Injection (440mg)
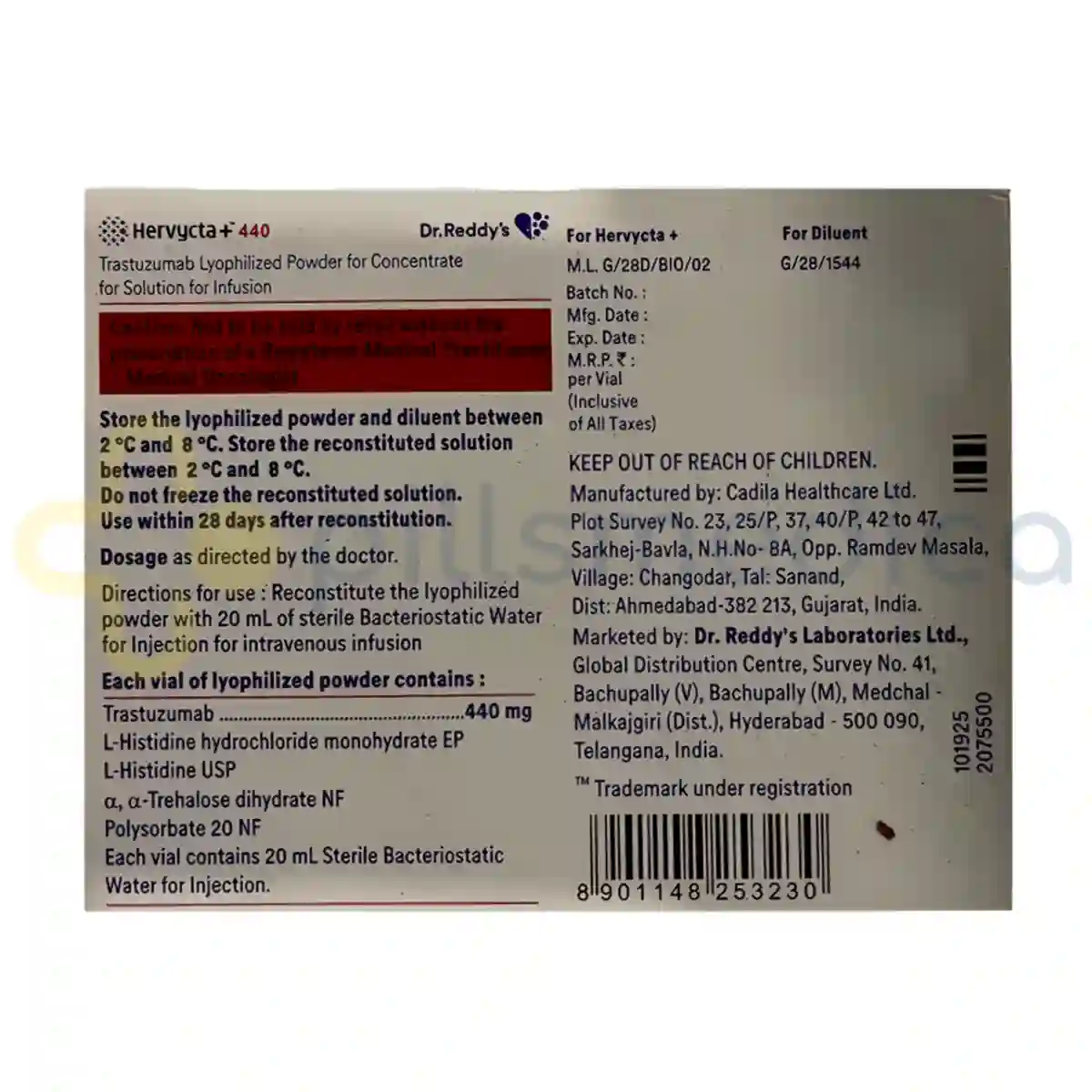
Hervycta Injection (440mg) should be stored in a refrigerator at a temperature between 2°C to 8°C (36°F to 46°F) and protected from light. It should not be frozen. Once prepared for administration, the injection should be used immediately or stored at room temperature for a limited time, as per the healthcare provider's instructions. Proper disposal of unused or expired medication should be done according to local regulations, and patients should not dispose of it in household trash or down the toilet.

Here are a few quick tips for Hervycta Injection (440mg)
To use Hervycta Injection (440mg) effectively and safely, patients should adhere to the prescribed treatment schedule, attend all follow-up appointments, and communicate openly with their healthcare provider about any side effects or concerns. Staying hydrated during treatment and following any dietary recommendations can also enhance the effectiveness of the medication. Patients should also keep track of their symptoms and report any significant changes to their healthcare team.

Parameters that should be monitored Hervycta Injection (440mg)
While using Hervycta Injection (440mg), healthcare providers should monitor several parameters, including cardiac function, liver and kidney function, and overall response to treatment. Regular blood tests may be necessary to assess the patient's health status and to detect any potential side effects early. Monitoring for infusion-related reactions during administration is also crucial to ensure patient safety.

Considerations related to diet Hervycta Injection (440mg)
Dietary considerations while using Hervycta Injection (440mg) may include maintaining a balanced diet rich in nutrients to support overall health and immune function. Patients should discuss any specific dietary restrictions or recommendations with their healthcare provider, especially if they are undergoing concurrent chemotherapy or have other health conditions that may require dietary adjustments.

Question and Answer (FAQ)
Q: What is Hervycta Injection used for?
A: Hervycta Injection is used to treat HER2-positive breast cancer and gastric cancer.
Q: How is Hervycta Injection administered?
A: Hervycta Injection is administered intravenously by a healthcare professional.
Q: What are the common side effects of Hervycta Injection?
A: Common side effects include fever, chills, nausea, vomiting, and fatigue.
Q: Can I take Hervycta Injection if I am pregnant?
A: Pregnant women should exercise caution and discuss the risks with their healthcare provider.
Q: Is it safe to breastfeed while using Hervycta Injection?
A: Lactating women should consult their healthcare provider, as Trastuzumab may be excreted in breast milk.
Q: What should I do if I miss a dose of Hervycta Injection?
A: Contact your healthcare provider immediately to determine the best course of action.
Q: What are the risks of overdosing on Hervycta Injection?
A: Overdosing may lead to severe side effects, including heart problems and infusion reactions.
Q: Can I drink alcohol while taking Hervycta Injection?
A: It is advisable to exercise caution with alcohol consumption, as it may affect overall health.
Q: How should I store Hervycta Injection?
A: Store Hervycta Injection in a refrigerator and protect it from light.
Q: What should I monitor while using Hervycta Injection?
A: Patients should monitor cardiac function, liver and kidney health, and report any concerning symptoms to their healthcare provider.






Reviews
There are no reviews yet.